The is Part II of my look at Changing World Technologies’ thermal depolymerization process. This essay came from a reader, and was originally posted on April 12, 2007.
But I also want to add some comments that regular reader “Optimist” added following the previous essay. First, those comments:
The 85% efficiency claim is based on a faulty mass balance. The faulty mass balance is the basis for an equally faulty energy balance. You can verify by comparing production data (bbl oil/ton of waste) to the mass balance (still) presented by CWT.
Contrary to what the breathless writers at Discover magazine believe, this technology is good only for recycling lipids (fats and oils) and the fat-soluble amino acids in protein. To understand why you need to follow the process flow diagram, which consists of three key steps:
1. Thermal Depolymerization (aka Dilute Acid Hydrolysis – yes, the process uses sulfuric acid).
2. Separation of water and fat/oil.
3. Decarboxylation of fatty acids to yield hycrocarbon (diesel) product.Anything soluble in water goes into the effluent in step 2. That includes (but is not limited to) all carbohydrate and the bulk of the protein hydrolysis product (amino acids).
CWT cleverly states that this makes the effluent a high quality fertilizer. Probably true. But that high quality fertilizer contains BTUs not available as fuel (the main product).
Another comment from Optimist:
To their credit, Discover magazine did raise another issue: product quality: Fuel quality was another challenge. Changing World Technologies‘ thick, tarry fuel resembles boiler-grade fuel oil. One prospective buyer insisted on what the company called “unacceptable pricing terms” for its relatively unproven product. In the end, CWT sold only 93,000 of the 391,000 gallons of fuel it produced and earned just 99 cents for each one. At the time, wholesale fuel oil distributors were raking in $2.50 to $3.30 per gallon. Even with the $1-per-gallon U.S. biofuels tax credit for every gallon sold, Changing World Technologies paid more for Butterball’s turkey offal than it earned back in revenue. (Accounting for all its operating costs, the company lost $5,003,000 in the first quarter of 2008, though operating at a loss is not uncommon or necessarily a very bad sign for a technology startup.) Emphasis added.
Don’t worry – I’m sure next year they’ll be printing money…
In light of this, I am not sure why they think it’s a good idea to do an IPO now.
Now for the essay from a reader who provided some very specific details on what went wrong. He included a presentation in which he referred to several slides, and I will pull those out and post them so the references are clear. I will also insert some comments in the text [like this].
—————————————
Robert,
I enjoy your blog quite a lot. Intelligent analysis is rare. Coupled with unbiased interpretation it is almost an unknown.
Saw your discussion of TDP/TCP. Pretty much spot on. As a chemical engineer I thought you’d be interested in some deeper insights of how the process works. This is all information that used to be available on the web, but most of it has been removed.
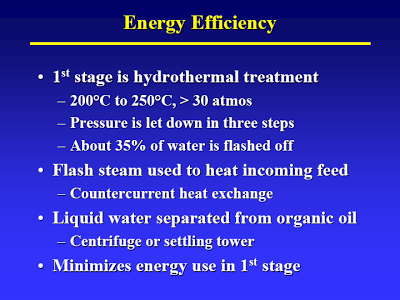
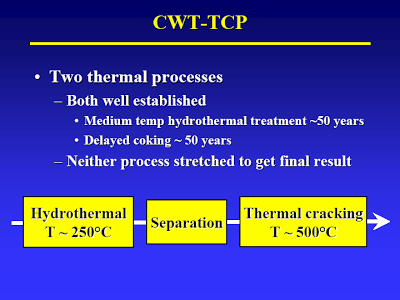
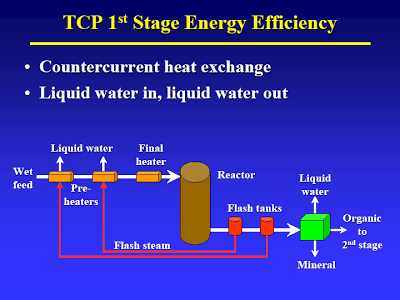
Start with the lecture (attached) by Terry Adams, CWT technical officer at MIT in April 2005 – best TDP technical article I know of [I have searched for an online version of this, but to no avail. Perhaps using the Wayback Machine one could locate an online archive of the original presentation]. The way I understand it, the process basically consists of two thermal treatment steps. The first step (slide #13) is a low temp/high pressure step that causes hydrolysis of all the biological material. A check of steam tables confirms that pressure is just high enough to maintain liquid water at the temperature given.
The first stage is followed by separation (slide #3).
As indicated in slide #14 they have a clever way of flashing off some of the water and then using the steam to heat the feedstock [This sort of heat recovery is standard practice in the petrochemical industry]. This is at the heart of their claims about high efficiency: the steam is condensed, so most of the water in the feedstock is discharged as liquid. Calling it distilled water, is of course salesmen talk that would make a used car salesman’s eye’s water.
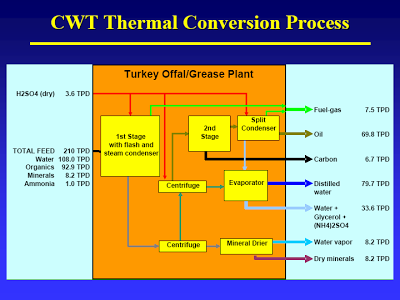
But take a closer look: After separation only the “organic oil” goes to the second stage. After full hydrolysis (let’s just assume that for now) what monomers would be part of the organic oil? Fatty acids barely make it into this oil, due to the little known fact (see flow diagram on slide #11) that sulfuric acid is used to aid hydrolysis [If I had known that, I had forgotten about it. That does put quite a different spin on the whole process]. (DOE would call the first stage by another name: Dilute Acid Hydrolysis). Some fat-soluble amino acids. That’s it. (I bet you can figure out what cellulose fed to these two units would yield…) [It would interesting to see some yields on this. What I would really like to see is what they get if they threw corn in there. If their energy balances are really good – and even with all that has gone wrong they appear to be better than for corn ethanol – then I would like to see some experiments in that direction.]
Of course, CWT are master salesmen. The water-soluble amino acid and glycerol solution is not waste: it is a wonderful liquid fertilizer (slide #23). Talk about taking a lemon and making lemonade…
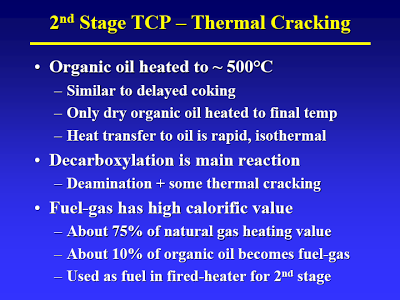
So, the “organic oil” goes to the second stage (high temperature/low pressure) where the fatty acids are decarboxylized (to yield oil) and some of the amino acids are deaminated and decarboxylized to yield who-knows-what (slide #15, point 2).
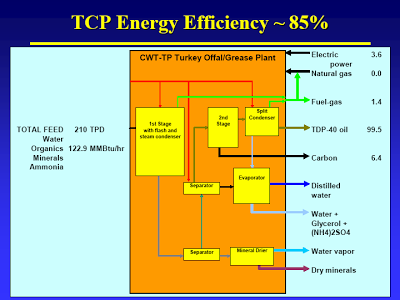
You raise the question of how on earth did CWT get their cost estimates so wrong. Well, a large factor in that would be overestimating yield (and per extension efficiency). CWT has long claimed that TDP has an energy efficiency of 85% (heading slide #12). Right there you smell a skunk. Now the dirty details.
The mass balance, slide #11 [posted earlier], shows that CWT probably did not take the CO2 that results from decarboxylation into account. This causes them to overestimate fuel production. You can easily do the calc’s I’m sure, but it is spelled out here. Apologies for the format, got mangled when they changed their format [That thread was a very good discussion on this issue; perhaps I will pull it out, reformat it, and post it at some point].
The energy balance, slide #12, does not include the energy present in the “liquid fertilizer”. What, all that glycerol and amino acids contain no energy? The water vapor also presents energy lost, even if it’s not much.
The mass and energy balances actually date from a previous publication (February and March 2004), also attached. One would expect that CWT would have discovered the error in the interceding year, and corrected it. I guess they were to busy ironing out the substantial start-up problems, such as the odor issue, you mentioned.
You may have notice a subtle shift between those two breathless Discover articles. Instead of producing 500 bbl from 210 tons of waste (first article), they now need 290 tons (20 tons of it pure pig fat), or a 28% reduction in oil yield. Instead of claiming 2.4 bbl/ton of waste, it is now 1.7 bbl/ton (validating an estimate of the maximum yield of ~2.0 bbl/to). Funny thing is Appel and his team still use the 2.4 figure in their financial analysis, even when it would help their argument to use the 1.7 real number. From the second Discover article: “‘We thought we would get $24 a ton for taking the waste,’ says Appel. ‘Instead we are paying $30 a ton.’ That alone raises his production costs about $22 a barrel.” How did they get to $22? ($24/ton + $30/ton)/2.4 bbl/ton = $22.50/bbl. Using the real number would yield: ($24/ton + $30/ton)/1.7 bbl/ton = $32/bbl. Also getting less yield would raise production cost in a number of ways, including the fact that they may be buying natural gas for heating…
So where does that leave TDP? No doubt it is not the silver bullet once claimed. None of the “anything” into oil that seduced Discover’s reporters. And costs are substantial. However, it seems like a good process for converting waste grease into liquid fuel. Much better than say biodiesel. Look at the feedstock (slide #6). How much cleaning (i.e. money and energy) would that stuff need to make it suitable as feedstock for a biodiesel plant? TDP uses sulfuric acid, whereas biodiesel uses methanol and a catalyst (usually NaOH). In terms of energy and money, I suspect TDP has the better input here. TDP yields a liquid fuel that is chemically almost identical to fossil diesel (without the sulfur and aromatics). TDP-40 can be blended with diesel in any ratio 1 to 100, without any issues. As Minnesota discovered last winter, biodiesel has some issues with cold weather. [Having worked in a Montana refinery, I can attest to the fact that winter properties for diesel are critical. I am aware that biodiesel has some problems with pour and cloud points in cold weather, limiting their usage to small blend fractions.]
The main threat to TDP, as I see it, is a process developed by Neste Oil, Finland, that I read about at GCC. Apparently this process allows an existing refinery to incorporate waste grease as a feedstock, without a radical change to the process (or a brand new SS plant). Even that process is not a slam-dunk, as I’ve seen reports of canceled projects.
So yes, you nailed it: these guys overpromised and underdelivered big time. But in terms of the big picture I give them some credit: at least we are not talking about food -> fuel (as with most of the biodiesel plants being built in Europe, proving that the food -> fuel madness is not endemic to North America). [Oh, I agree completely. It is not the process that I took issue with; in fact I do applaud their initiative. My concern was the completely willingness of so many to accept this as the solution to our energy problems. I see the same thing happening right now with cellulosic ethanol.] They probably help to advance the debate on waste -> energy quite a bit. And they do have a working plant, which is more than we can say about Washington’s next big thing, aka cellulosic ethanol. [I will probably write the same article on cellulosic ethanol in just a few years – overpromised and underdelivered. I see many parallels here.]


thanks, i needed that to bring me back to a better point of objectivity. i didn’t grasp all the technical nooks/crannies, but enough together with the macro-message.
i’ll continue to track production quality/volume/cost/usage of several cos with BIG OIL sponsors in the “cellulosic” arena. should they commit big $$$, i’ll look deeper.
fran
Hey Robert, thanks for your words the other day and letting me post here, I was just very pissed that I was banned from TOD for no good reason, annoying. Anyway, not sure if you saw this yet:
http://www.greencarcongress.com/2009/01/biosyncrude-gas.html#more
Biosyncrude Gasification Process Could Produce Motor Fuel at Cost of Around $3/gallon
Thoughts?
Cheers
TheAntiDoomer
Also reported on Greencarcongress (a great site, btw) is a new study on corn ethanol, long a villain in these quarters. The study claims:
“The net energy ratio (of corn ethanol), which averaged 1.2 to 1 in earlier studies, is 1.5-1.8 to 1 in the recent research, Cassman said. That means that for every unit of energy it takes to make ethanol, 1.5 to 1.8 units of energy are produced as ethanol.
Even more striking is the corn ethanol’s potential to replace oil. This new study estimates that 10-19 gallons of ethanol are produced for every gallon of petroleum used in the entire corn-ethanol production life cycle. The range in the ethanol-oil replacement value, as well as the ranges measured for net energy efficiency and GHG emissions reduction, are due to differences in crop management practices and ethanol plant performance. “
The study was Jan 24 on GCC, and was done by University of Nebraska guys,
I would like some smarties to take a look at the study. The statement that ethanol replaces about 10-19 gallons of oil is fascinating, if true. After all, we do not have an energy problem, we have an oil problem (it is imported from unreliable thug states).
Anti-Doomer; You are fighting the tide at TOD. Forget about it. If they were a “forum,” they would invite people with different viewpoints to participate. I remain extremey dubious about the motivations of TOD. It doesn’s pass the smell test.
Biosyncrude Gasification Process Could Produce Motor Fuel at Cost of Around $3/gallon
TAD, those costs seem pretty reasonable. The energy efficiency they report is realistic based on what I know about GTL, CTL, and BTL.
I was pleased to see use of “XTL” to generically to refer to gas, coal, and biomass to liquids. To my knowledge I was the first to coin that term in this 2006 essay.
By the way, you are probably aware of the site, but Peak Oil Debunked is also a gathering place for TOD refugees. While the site owner, JD, has not been banned from TOD (at least not yet), you can see in the comments that some of his readers have been. JD is sort of like you, in that he will post more upbeat stuff, while ‘debunking’ the idea that we are all doomed due to peak oil.
Cheers, RR
P.S. I told Nate yesterday that I am going to take a little break from TOD. Just too much on my plate right now.
@ TAD
What thread were you posting in when the ban occurred ?
BTW.
My neighbor is a grad student at UNL – good guy and a 4.0 student – and I asked him if he had heard any more about E3.
He said he had no update. E3 was a client of his, as part of their product went to meat production.
His earlier statement to me was a boiler that was provided by a contractor had blown up. That was public info. He also indicated the contractor was very small and it would be like getting a blood out of a turnip in regard to compensation from the ensuing litigation.
Back to square #1 in Mead NE.
RBM
As I’ve stated before, I think E3 made things way too complicated. Too much interdependence. Something is bond to go wrong in a system like that.
I would propose the following improvement: get rid of the ethanol. Feed the cows corn, put the manure in an anaerobic digester, use the biogas wisely (perhaps GTL, now that there are modular technologies for this).
Ethanol is just not a good enough fuel to put at the center of any biofuel scheme.
“I would like some smarties to take a look at the study.”
Benny,
You don’t have to be especially smart to see something is cockeyed with that study.
Any process that returned 180% more energy than it consumed would be self-sustaining and would need no external energy inputs.
If that was really true of making ethanol, why would corn farmers and ethanol plants ever want to use fossil fuels?
If making ethanol does actually return 180% more energy than it consumes, and they continue using fossil fuels anyway, they aren’t very bright.
Wendell you may want to hold the sarcasm and open you mind to alternatives. My wood pile is 3000% reduction in fossil fuels. The contractors remodeling nest door were going to haul the wood scrape to the dump. I have not bought fire wood in years. Just by collecting wood that is destined to produce ghg gas and using it to heat my house. This does not count the energy lost if I belong to a health club and got my exercise by driving to someplace to exercise. Using to wood heat is not new.
The grand daddy of all is the approximately bazillion % gain from making electricity with weapons grade uranium. Not a small source, more than 10% of US electricity is generated
More mundane is AD. It is only a about 900% efficient when electricity is produced.
Industrial Ecology is about saving energy used to process waste by using it as a feedstock for a different process. In nature, bacteria break down the waste and store the energy and nutrients in the bacteria cells. Later other small animals that live in the soil eat the bacteria and release the nutrients to the plants. This is called the soil food web.
One dairy farm AD produced twice as much energy as was possible based on the VOC in the manure. The farm has to add a second generator. The source of the carbon was shreded paper used for bedding. Cows produce excess enzymes that break down the cellulose in papers. The paper provides nucleation points making increasing the growth rate of bacteria in the AD.
The organic fertilizer increased the efficiency of chemical fertilizer for growing corn by 200% while reducing wind and water erosion (which may explain improved crop yields). More corn is produced and the energy is separated to produce ethanol and animal feed. If there is an issue with Industrial Ecology it is that it is too complicated.
So Wendell if EROI calculation sound silly it is because they are simplistically silly. If your pollution problems is blowing dust, the root cause of wind erosion is lack of organic matter binding the soil together. AD of manure produces compost to solve that problem. How do you factor that into EROI.