I am still working on an essay on butanol, pentanol, and higher-chain alcohols, but it has been really difficult to find the information I am looking for. (But I have figured out why bio-butanol has not taken off). Regardless, I hope to complete it in a few days.
In the interim, in response to my TDP essay, I received a comprehensive e-mail from a reader who provided some very specific details on what went wrong. He included a presentation in which he referred to several slides, and I will pull those out and post them so the references are clear. I will also insert some comments in the text [like this].
—————————————
Robert,
I enjoy your blog quite a lot. Intelligent analysis is rare. Coupled with unbiased interpretation it is almost an unknown.
Saw your discussion of TDP/TCP. Pretty much spot on. As a chemical engineer I thought you’d be interested in some deeper insights of how the process works. This is all information that used to be available on the web, but most of it has been removed.
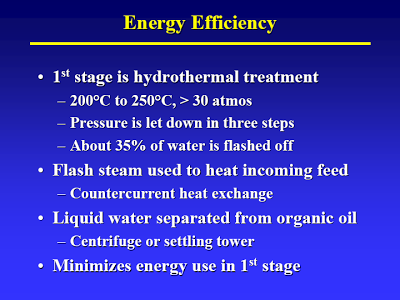
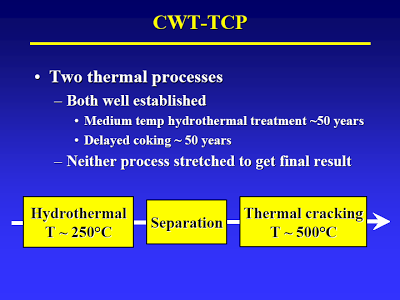
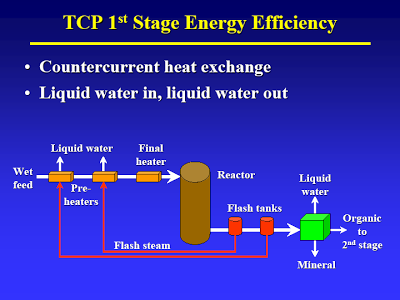
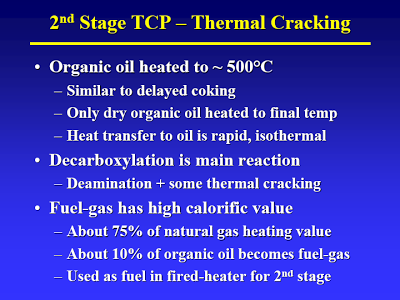
Start with the lecture (attached) by Terry Adams, CWT technical officer at MIT in April 2005 – best TDP technical article I know of [I have searched for an online version of this, but to no avail. Perhaps using the Wayback Machine one could locate an online archive of the original presentation]. The way I understand it, the process basically consists of two thermal treatment steps. The first step (slide #13) is a low temp/high pressure step that causes hydrolysis of all the biological material. A check of steam tables confirms that pressure is just high enough to maintain liquid water at the temperature given.
The first stage is followed by separation (slide #3).
As indicated in slide #14 they have a clever way of flashing off some of the water and then using the steam to heat the feedstock [This sort of heat recovery is standard practice in the petrochemical industry]. This is at the heart of their claims about high efficiency: the steam is condensed, so most of the water in the feedstock is discharged as liquid. Calling it distilled water, is of course salesmen talk that would make a used car salesman’s eye’s water.
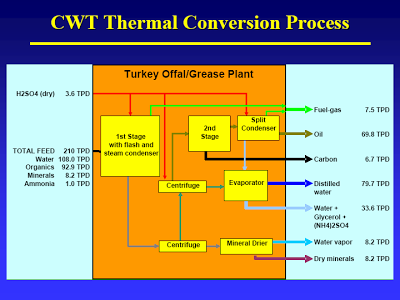
But take a closer look: After separation only the “organic oil” goes to the second stage. After full hydrolysis (let’s just assume that for now) what monomers would be part of the organic oil? Fatty acids barely make it into this oil, due to the little known fact (see flow diagram on slide #11) that sulfuric acid is used to aid hydrolysis [If I had known that, I had forgotten about it. That does put quite a different spin on the whole process]. (DOE would call the first stage by another name: Dilute Acid Hydrolysis). Some fat-soluble amino acids. That’s it. (I bet you can figure out what cellulose fed to these two units would yield…) [It would interesting to see some yields on this. What I would really like to see is what they get if they threw corn in there. If their energy balances are really good – and even with all that has gone wrong they appear to be better than for corn ethanol – then I would like to see some experiments in that direction.]
Of course, CWT are master salesmen. The water-soluble amino acid and glycerol solution is not waste: it is a wonderful liquid fertilizer (slide #23). Talk about taking a lemon and making lemonade…
So, the “organic oil” goes to the second stage (high temperature/low pressure) where the fatty acids are decarboxylized (to yield oil) and some of the amino acids are deaminated and decarboxylized to yield who-knows-what (slide #15, point 2).
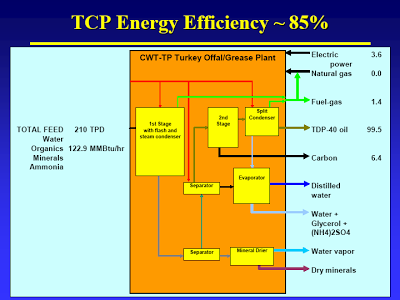
You raise the question of how on earth did CWT get their cost estimates so wrong. Well, a large factor in that would be overestimating yield (and per extension efficiency). CWT has long claimed that TDP has an energy efficiency of 85% (heading slide #12). Right there you smell a skunk. Now the dirty details.
The mass balance, slide #11 [posted earlier], shows that CWT probably did not take the CO2 that results from decarboxylation into account. This causes them to overestimate fuel production. You can easily do the calc’s I’m sure, but it is spelled out here. Apologies for the format, got mangled when they changed their format [That thread was a very good discussion on this issue; perhaps I will pull it out, reformat it, and post it at some point].
The energy balance, slide #12, does not include the energy present in the “liquid fertilizer”. What, all that glycerol and amino acids contain no energy? The water vapor also presents energy lost, even if it’s not much.
The mass and energy balances actually date from a previous publication (February and March 2004), also attached. One would expect that CWT would have discovered the error in the interceding year, and corrected it. I guess they were to busy ironing out the substantial start-up problems, such as the odor issue, you mentioned.
You may have notice a subtle shift between those two breathless Discover articles. Instead of producing 500 bbl from 210 tons of waste (first article), they now need 290 tons (20 tons of it pure pig fat), or a 28% reduction in oil yield. Instead of claiming 2.4 bbl/ton of waste, it is now 1.7 bbl/ton (validating an estimate of the maximum yield of ~2.0 bbl/to). Funny thing is Appel and his team still use the 2.4 figure in their financial analysis, even when it would help their argument to use the 1.7 real number. From the second Discover article: “‘We thought we would get $24 a ton for taking the waste,’ says Appel. ‘Instead we are paying $30 a ton.’ That alone raises his production costs about $22 a barrel.” How did they get to $22? ($24/ton + $30/ton)/2.4 bbl/ton = $22.50/bbl. Using the real number would yield: ($24/ton + $30/ton)/1.7 bbl/ton = $32/bbl. Also getting less yield would raise production cost in a number of ways, including the fact that they may be buying natural gas for heating…
So where does that leave TDP? No doubt it is not the silver bullet once claimed. None of the “anything” into oil that seduced Discover’s reporters. And costs are substantial. However, it seems like a good process for converting waste grease into liquid fuel. Much better than say biodiesel. Look at the feedstock (slide #6). How much cleaning (i.e. money and energy) would that stuff need to make it suitable as feedstock for a biodiesel plant? TDP uses sulfuric acid, whereas biodiesel uses methanol and a catalyst (usually NaOH). In terms of energy and money, I suspect TDP has the better input here. TDP yields a liquid fuel that is chemically almost identical to fossil diesel (without the sulfur and aromatics). TDP-40 can be blended with diesel in any ratio 1 to 100, without any issues. As Minnesota discovered last winter, biodiesel has some issues with cold weather. [Having worked in a Montana refinery, I can attest to the fact that winter properties for diesel are critical. I am aware that biodiesel has some problems with pour and cloud points in cold weather, limiting their usage to small blend fractions.]
The main threat to TDP, as I see it, is a process developed by Neste Oil, Finland, that I read about at GCC. Apparently this process allows an existing refinery to incorporate waste grease as a feedstock, without a radical change to the process (or a brand new SS plant). Even that process is not a slam-dunk, as I’ve seen reports of canceled projects.
So yes, you nailed it: these guys overpromised and underdelivered big time. But in terms of the big picture I give them some credit: at least we are not talking about food -> fuel (as with most of the biodiesel plants being built in Europe, proving that the food -> fuel madness is not endemic to North America). [Oh, I agree completely. It is not the process that I took issue with; in fact I do applaud their initiative. My concern was the completely willingness of so many to accept this as the solution to our energy problems. I see the same thing happening right now with cellulosic ethanol.] They probably help to advance the debate on waste -> energy quite a bit. And they do have a working plant, which is more than we can say about Washington’s next big thing, aka cellulosic ethanol. [I will probably write the same article on cellulosic ethanol in just a few years – overpromised and underdelivered. I see many parallels here.]


This was a great simplified explanation of the process. I think I misunderstood your first article’s intent and if I can sum up your point as I understand it:
If an experienced and qualified team went at TDP, it has a potential for efficient recycling.
Would you agree?
If an experienced and qualified team went at TDP, it has a potential for efficient recycling.
It has the potential for that now, even with the team they have in place. I think it’s an interesting and useful process, and an example of the kind of innovation that will be critical in the years ahead.
The lesson here is how not to get carried away by assuming this is going to be a major solution to our energy needs. I see so many parallels here with cellulosic ethanol.
Cheers, Robert
I bet you can figure out what cellulose fed to these two units would yield…
Not being a chemical engineer, I for one have no idea. What would it yield?
Hydrolysis of cellulose yields glucose. Glucose is a great substrate for ethanol fermentation (or any biological growth). This is the direction most of the cellulosic ethanol research are taking: hydrolyze the cellulose using heat and acid, then ferment the resulting glucose to yield ethanol. The challenges include:
1. You need severe reaction conditions to hydrolyze cellulose. Those tend to breakdown the glucose before ethanol is produced.
2. Only part of the biomass is cellulose, and only part of that is converted to glucose. Much of the feedstock goes to waste.
In the context of TDP the point is that glucose is water soluble, which means it ends up in the “liquid fertilizer”, aka “sterile” or “distilled” effluent. In other words, none of the cellulose (or any other carbohydrate) makes it into the fuel product. So much for ANYTHING into oil…
Robert, great article. I think I found the original presentation but the slides doesn’t quite match up with the slides posted. I can’t seem to post a link right now but if you do a Google search for “TDP MIT Terry Adams” it shows up as my third choice down.
Looks like I jumped the gun there, that presentation is more general and not about TDP.
Robert, if you’ll send me that presentation I’ll find a home for it.
I have to admit, seeing this gave me a bit of a pause. Am I another one of those cranks, only one level beyond “don’t know what conservation of energy means”? I’d like to think not, and it is certainly possible that there are serious roadblocks in all four of my candidate energy conversion technologies. On the other hand, it really does take only one to make it.
I think the Neste Oil process referred to is called NEXBTL.
You can find a description (their own overview) of the process at:
http://www.climatechange.ca.gov/events/2006-06-27+28_symposium/presentations/CalHodge_handout_NESTE_OIL.PDF
http://bioenergy.ucdavis.edu/materials/NExBTL%20Enviro%20Benefits%20of%20paraffins.pdf
and Neste Oil own presentation about benefits of their process:
http://www.energy.ca.gov/bioenergy_action_plan/documents/2006-03-09_workshop/2006-03-09_NESTE_OIL.PDF
As for the cancellations…
I think they cancelled one co-project with the French Total due to plant cost issues (plant was supposed to be built in France).
I think they are in discussion with OMV (AT) on building NExBTL plants.
Neste Oil has almost finished building first of the two new process plants for NEXBTL process in Finland. Plant cost is c. 100 MEUR / plant.
Refining capacity is claimed to be 170 000 metric tons of biodiesel per year (per one plant).
As for how good the process is, I’m not qualified to analyze it.
I’m not affiliated with them in any way. I just know all this, because I live near where they operate and you can’t dodge the news 🙂
Cambridge Prof. David MacKay has been working on “Sustainable Energy – Without the Hot Air” and a .pdf draft is available here:
http://www.withouthotair.com/
http://www.inference.phy.cam.ac.uk/mackay/
He has laid out the book with the idea of a balance sheet of Consumption/Production and goes through the details of the existing schemes. I found even the draft to be worth reading.
Refining capacity is claimed to be 170 000 metric tons of biodiesel per year (per one plant).
This is where it gets a tad confusing: like TDP, the NExBTL process produces hydrocarbons (similar to fossil diesel) and not methyl esters (traditional biodiesel). ECN tried to call their product (also hydrocarbon produced from biomass gasification/Fischer-Tropsch) a green diesel, but that term has already been used for good old fossil fuel in the US of A.
Plant cost is c. 100 MEUR / plant.
A 170,000 metric tons of biodiesel ~ 1,275,000 bbl. That’s about EU28,600 or $21,200 per installed barrel (Am I doing this right, Robert?)
That’s not half bad compared to: According to the EIA’s Annual Energy Outlook 2006, capital costs are $15,000-20,000 per installed barrel for a conventional oil refinery, $20,000-$30,000 for an ethanol plant, around $40,000 for gas-to-liquids (GTL), around $60,000 for coal-to-liquids, and around $120,000-$140,000 for biomass-to-liquids.
That’s about EU28,600 or $21,200 per installed barrel (Am I doing this right, Robert?)
WHOOPS! Got the currency conversion part wrong. EU28,600 would be $38,700 per installed barrel (Would it be cheaper in USA than EU?). Quite a bit more expensive than a conventional refinery, about the same as GTL. But at least the feedstock is free.
I thought something smelled funny about TDP long before the current problems came to light.
I am not a chemist, but I was immediately suspicious of the “anything into oil” claim. First of all, it generally seems too good to be true. More specifically: I can well believe you can make oil out of rendered fat and other low-grade organic waste. But I have a very hard time believing that any process can make oil out of, say, old tires in an efficient fashion. Like I said, I am not a chemist, but the idea of turning a highly-stabilized, heavily interlinked solid polymer into a liquid, relatively short-chain polymer possessing significant chemical potential energy just seemed intuitively wrong.
So my question for you is twofold: Does your knowledge of chemistry support my intuition as sound? And, relatedly, do you believe this process could be used to transform something like old tires into a useful endproduct? If so, it might have some value as a waste disposal system, even if it didn’t produce alot of useful oil.
A poster at TOD called these schemes “Alchemy” and that sums it up.
Lead into gold.
Turkeys, tires and corn stover into oil.
“Ladies and gentlemen, attention please. Come in close so everyone can see. I got a tail to tell and listenin’ won’t cost a dime….”
— Snake Oil, Steve Earl
http://steveearle.net/lyrics/ly-coppe.php#snakeoil
The last verse is the best:
“Well ain’t your President good to you
Knocked ’em dead in Libya, Grenada too
Now he’s taking his show a little further down the line
Well, ‘tween me and him people, you’re gonna get along just fine”
Well, fom the presentation it is clear that the Carthage plant was designed specifically to convert lipids (biological fats and oils) into hydrocarbons (diesel). So that plant would probably not be able to convert rubber or plastic waste into fuel.
However, it seems CWT has done some research work on converting automotive shredder residue (ASR) into oil. ASR is the gunk that is left after they shredded up an old car and recovered the valuables, mostly the metal.
So, the question is: Do we expect this to work? In the absence of real world data one can only speculate. So here goes: Bear in mind that plastics have the generic formula of CH3-(CHR)n-CH3, where n would be several thousands or tens of thousands. R would differ from plastic to plastic, R=H for polyethylene, R=CH3 for polypropylene, etc.
According to Wikipedia: The average chemical formula for common diesel fuel is C12H26, ranging from approx. C10H22 to C15H32. In reality, diesel is a witch’s brew of all sorts of compounds. Nonentheless, the bottom line is this: break the plastic up in C10 – C15 pieces, and violá, you have diesel.
Tire rubber contains one other complication: it is vulcanized. The link shows the chemical structure – it is basically uses a poly-S bridge to connect separate polymer strands together, giving the rubber its good physical properties. So, to convert tire rubber into oil, you’d also have to strip out the sulfur. Remember the neigbor’s complaint, odors?
Can TDP do this? The fact is that they have some publications to that effect. As explained, the chemistry would not be that involved. More-over, there are related processes that seem to focus on precisely this conversion (plastic to liquid fuel). Unfortunately, they also feel compelled to repeat the ANYTHING into oil BS claim.
However, this process is very different from what they do at Carthage, so the set-up would probably look different and operate at different temperatures and pressures.
If so, it might have some value as a waste disposal system, even if it didn’t produce alot of useful oil.
I think it is all or nothing. If it works on plastic/rubber, yields should be pretty high. Unlike lipids (or biomass in general) the plastic/rubber feedstock contains mostly hydrogen and carbon, like the oil product. The feedstock energy content would also be higher than that of biomass.
BTW, one of the technical papers on TDP is still available on the web. Bear in mind this was published before they had the plant in operation, so it describes the conditions (yields, efficiencies, etc.) that they thought they would get, as opposed to what they are actually getting.
FYI, I’ve hosted the presentation and two other documents at ergosphere.wordpress.com.
One significant factor: this chemistry is done at high temperature in water. If you break a long -(CH2)-(CH2)- chain and add water, you can get -(CH2)OH and -CH3. Robert can correct me, but I see no inherent problem with breaking up long chains such as polyethylene into short hydrocarbons and alcohols via hydrolysis.
The sulfur issue with vulcanized rubber depends on how it comes off during the breakup. If it combines with the hydrocarbon chains, it would require hydrodesulfurization; if it comes off neatly as H2S, it isn’t part of the fuel and can be turned into gypsum relatively easily.
Well, TDP isn’t entirely dead:
HOUSTON and SPRINGDALE, Ark., April 16, 2007 — ConocoPhillips and Tyson Foods, Inc. [NYSE:TSN] will announce a strategic alliance at 12 p.m. CDT today to produce and market the next generation of renewable diesel fuel, which will help supplement the traditional petroleum-based diesel fuel supply. The alliance plans to use beef, pork and poultry by-product fat to create a transportation fuel. This fuel will contribute to America’s energy security and help to address climate change concerns.
Using a proprietary thermal depolymerization production technology, the animal fats will be processed with hydrocarbon feedstocks to produce a high-quality diesel fuel that meets all federal standards for ultra-low-sulfur diesel. The addition of animal fat also improves the fuel’s ignition properties, while the processing step improves its storage stability and handling characteristics.
Investments made by ConocoPhillips and Tyson will allow for the processing and handling of fat and enhance the ability of the United States to produce energy from a variety of sources, including domestically-produced vegetable oils.
The processing technology was developed at ConocoPhillips, culminating in a successful test at the company’s Whitegate Refinery in Cork, Ireland. ConocoPhillips began commercial production of renewable diesel using soybean oil in Ireland late last year.
I know the guys who developed the Whitegate process. I think one of the problems with bio fuels is that people have tried to develop them as stand alone processes. It makes more sense to me to integrate biofuels into the refining process to take advantage of similar process capability, waste heat, coproducts and other advantages.
One of my areas of research was copdroducing an ethanol-gasoline mixed blendstock. We used gasoline components to break the ethanol-water azeotrope, and low pressure waste steam to provide the heat for the distillation.
Unfortunately, the U.S. tax subsidy only applies to making ethanol “neat” – which makes no sense unless you DON’T want the competition from refiners. The ethanol blend would need to be stored differently and could not be sent out with the pipeline. But the refinery I worked at sold a fair amount of gasoline over the rack which could be blendeded to E10.
Well, TDP isn’t entirely dead:
I wouldn’t really call that TDP (even though they referred to it that way in the press release). It is a different process than CWT’s.
I like this move. It has been in the works for a while – well you know how long ago the Whitegate results were announced. But since they are turning animal fat into biodiesel, they need to start gathering up the human variety at all the liposuction clinics. There is probably a fair amount. 🙂
Cheers, Robert
I’m listening to the press conference. They will be processing beef tallow from the Tyson Amarillo beef processing plant to the Borger refinery. That makes sense. Amarillo is one of the larger plants in the world. Transportation costs will be low to Borger.
I wouldn’t really call that TDP
No, not the same, we do the “T” part really well. We turn crappy Mayan crude and Venezuealan heavy oil into gasoline and diesel. Compared to that, animal fats seem easy.
Funny coincidence, I was tutoring my daughter and a few of her classmates in 8th grade chemistry last week. The sections we covered included plastic polymers, refining, starches, and lipds. We talked about how alkenes and styrenes are the monomers for plastics, sugars are monomers for starches, and amino acids for lipids.
I’m not sure the girls shared my enthusiasm for chemistry.
Lew Burke just did a really good job explaining how the process breaks oleic acid into two molecules of diesel, one molecule of propane, CO2 and water.
I was wondering why they call this process TDP. When did lipid hydrolysis become “depolymerization”? From what they describe, the process sounds a lot closer to NExBTL.
Now I know! How could I have forgotten?
I think this exposes one of the energy legislation’s most serious shortcomings: it names specific technologies, rather than setting a broad goal and describing how new technologies would qualify for subsidy.
Of course, if you are a lobbyist, you’d want to name specific technologies, since every new technology would have to hire a lobbyist…
Sorry – I didn’t explain it well. I was triple-tasking. Eating my lunch, listening to the press conference and typing here.
I may have confused you with my comment about daughter’s chemistry.
The process will take diglycerides and triglycerides in animal fat in the presence of a catalyst and hydrogen breaking the glycerin bonds to get either 2 or 3 molecules of fatty acid and one molecule of glycerin, which is converted to propane. The catalyst breaks the remaining fatty acids down to parrafins in the diesel boiling range, producing a saturated diesel with no sulfur and high cetane index.
You said it sounds like Neste’s process. Similar perhaps in that the end results are the same. There was a specific question about patents. The tech guy classified the process as “prior art”.
As I was trying to say: Now I know!
I guess I need to explain that the Energy Policy Act of 2005 includes this phrase:
“Renewable diesel defined – The term ‘renewable diesel’ means diesel derived from biomass (as defined in section 45K(c)(3)) using a thermal depolymerization process which…”
See section 1346 starting on page 1445.
The process will take diglycerides and triglycerides in animal fat in the presence of a catalyst and hydrogen breaking the glycerin bonds to get either 2 or 3 molecules of fatty acid and one molecule of glycerin, which is converted to propane. The catalyst breaks the remaining fatty acids down to parrafins in the diesel boiling range, producing a saturated diesel with no sulfur and high cetane index.
King,
Coverting glycerol to propane would be reduction, requiring (most likely) hydrogen. That sounds exactly like NExBTL. Perhaps NExBTL reduces -COOH to -CH3 (using hydrogen), while TDPv2 (or whatever) just converts -CH2-COOH to -CH3 + CO2 (not requiring hydrogen). Other than that, sounds quite similar…
We talked about how alkenes and styrenes are the monomers for plastics, sugars are monomers for starches, and amino acids for lipids.
I’m sure that’s just a typo. You obviously know that it should be “amino acids for proteins…”
it should be “amino acids for proteins…
Yes, I meant proteins. Should have reviewed my post a little closer.
I found it funny that between synthetic and organic polymers they stuck a section on petroleum refining.
Hi, I’m kinda late to the party here, but I wonder if the TDP process could be modified to remediate the black water sludge ponds from coal processing. There’s a tremendous amount of coal-bearing sludge in those ponds, and I’d like to see someone make a profit from cleaning them up…