Introduction
I have this neat new cellulose conversion process. I am looking for funding and working on a patent application. The invention is a personal cellulosic biomass reactor. In the first reaction step, the cellulose is partially converted to CO and H2 (syngas). In the second step, one could do many things with the syngas: produce methanol, ethanol, Fischer-Tropsch diesel, or combust it for heat or electricity. I chose the combustion for heat route, which occurs very rapidly following the 1st step. The combustion products are CO2 and water, but the CO2 that is released is equivalent to the CO2 that was taken up by the biomass while it was growing. It is therefore neutral with respect to Greenhouse Gas emissions. I am hoping to get some government subsidies, or possibly Silicon Valley startup money for my invention. You can see a picture of it below.
And there you have an example of how technical terminology and buzzwords can be used to confuse people. This is currently happening with cellulosic ethanol, so I thought I would write this essay to talk about the differences between cellulosic ethanol and biomass gasification.
What is Cellulosic Ethanol?
A popular trend in the media lately – encouraged by various ethanol advocates – is to liberally apply the “cellulosic” label. It has become a buzzword. This is the same thing that has occurred in the field of nanotechnology. Since lots of research funding is available for nanotechnology, things like ultra-fine powders are now being called nanotechnology. This trend is being driven, in my opinion, by a bid for some of the money flowing to the nanotechnology sector.
This brings us to some of the recent claims of a big breakthrough in “cellulosic ethanol” technology. However, one of the “breakthroughs” – biomass gasification – has been around for decades, and the technology is quite different from what is commonly denoted as cellulosic ethanol. It is not completely clear to me why some advocates are so eager to blur the distinction. Perhaps the law is written such that there is a danger of not receiving ethanol subsidies if a combustion process is used. Perhaps they want to be the first to claim commercial success of “cellulosic ethanol.” Perhaps they just want to give the public and the government the impression that great strides are being made in cellulosic ethanol technology, thereby encouraging more money to flow in that direction.
While cellulosic ethanol has only recently gained buzzword status, the term has been around for decades. The historical definition of the term implies certain particular process steps. There is some variance from process to process, but the things that are common are that the cellulose in the plant material is broken down into simple sugars, and then the sugars are fermented into ethanol.
More money than ever before is being poured into cellulosic ethanol, but there are multiple hurdles that have proven difficult to overcome. For a good layperson’s overview of the process, I recommend the recent article in the Chicago Tribune: Beyond corn: Ethanol’s next generation. I think the article paints a balanced picture of the technology. In brief, there are three major hurdles that have proven challenging to resolve.
The first is that plants have evolved defense mechanisms to prevent the cellulose from being easily broken down. Cellulose is actually a polymer – a long chain of connected sugars, and it is intermingled with hemicellulose and lignin. Cellulose provides structural strength to the plant walls. If it was easily broken down, microorganisms could attack the plants and limit their structural stability. What this means is that the cellulose must first be broken down with steam or a strong acid into component sugars that can be fermented, and this adds to the production costs. It is primarily this step that differentiates cellulosic ethanol from grain or sugarcane ethanol.
The second challenge is common to all ethanol fermentation processes, but not to gasification processes. The ethanol that is produced in a fermentation process is highly diluted with water. In fact, the ethanol produced from fermenting grain typically makes up only 15-20% of the solution, with the remainder being mostly water. For cellulosic ethanol, the picture is much worse. The crude ethanol in this case is typically less than 5%, with the remainder being water. Separating water and ethanol is a very energy-intensive process. Even where the EROEI is highly favorable, as is the case with sugarcane ethanol, the distillation step takes up a substantial amount of energy. While the distillation energy in the case of sugarcane is provided by burning the bagasse, separating out that much water is still a major energy sink.
The final challenge for cellulosic ethanol is that it takes a significant amount of biomass to produce the ethanol. As the nearby biomass is consumed, trucks have to travel farther to bring biomass to the refinery. This adds to the energy inputs, and worsens EROEI. According to the previously referenced Chicago Tribune article:
Richard Hamilton, CEO of Ceres Inc., Hamilton termed this “the tyranny of distance,” a major cost issue for would-be producers of cellulosic ethanol. If a refinery needs tons of biomass to produce fuel, he said, “by the end of the year you’re driving your truck a long way to get that wheat or corn stover.”
Some proponents don’t appreciate that there are multiple challenges in bringing cellulosic ethanol to market, and that these challenges won’t be easily solved. When asked about how long it would be before the challenges are resolved, Hamilton added:
“Trying to predict technology trends is a fool’s game,” he said. “I wish I could put my finger on just one bottleneck. But it doesn’t work that way.”
I don’t want to paint too grim a picture of the future for cellulosic ethanol. It is possible that all the hurdles will be overcome. But I also don’t want to present an overly optimistic scenario in which multiple bottlenecks are merely hand-waved away, and successful resolution is presumed. The challenges are well-understood. There just isn’t a clear path at this point to solving them all, and a process with multiple challenges will face a lower probability of success.
What is Biomass Gasification?
Biomass gasification is different from cellulosic ethanol in at least two major respects. First of all, it is a combustion process, not a fermentation process. As a combustion process, it can be self-sustaining once the combustion is initiated. It does not require continual inputs of energy as is the case with a fermentation process. The products of biomass gasification are syngas and heat, if the reaction is operated in an oxygen-deficient mode, or CO2 and steam (and much more heat) in the case where sufficient oxygen is supplied. In the case of the former, the syngas can be further reacted to make a wide variety of compounds, including methanol, ethanol, or diesel (via the Fischer-Tropsch reaction). A biomass gasification process followed by conversion to a liquid fuel is commonly referred to as a biomass-to-liquids (BTL) process.
However, there is one other major factor that differentiates biomass gasification from cellulosic ethanol. Biomass consists of a number of different components, including cellulose, hemicellulose, and lignin. In the case of cellulosic ethanol, only the cellulose and hemicellulose are partially converted after being broken down to sugars. The lignin and other uncoverted carbon compounds end up as (wet) waste, suitable for burning as process fuel only if thoroughly dried. Conversion is limited to those components which can be broken down into the right kind of sugars and fermented.
Gasification, on the other hand, converts all of the carbon compounds. Lignin, a serious impediment and waste product in the case of cellulosic ethanol, is easily converted to syngas in a gasifier. The conversion of carbon compounds in a gasification process can be driven essentially to completion if desired, and the resulting inorganic mineral wastes can be returned to the soil.
Gasification processes are of course not limited to biomass. In fact, biomass is currently the last feedstock of choice for economic reasons. It is much easier to transport natural gas and feed it on a continuous basis to a gasifier. In fact, most syngas in the U.S. today is made from natural gas. Coal is another option for gasification, and coal gasification is currently the dream of Montana Governor Brian Schweitzer.
While natural gas is easier to handle, and coal is cheaper, biomass is the only option capable of producing sustainable energy and mitigating greenhouse gas emissions. It is therefore the option that is most desirable, in my opinion. It is also a better option than most other “renewable” alternatives like corn ethanol or cellulosic ethanol. The conversion is much higher for gasification, and the energy return will undoubtedly be better because the product won’t need to be removed from an aqueous solution.
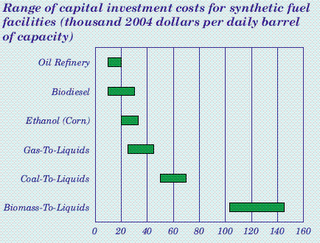
Compared to cellulosic ethanol, there are few technical challenges to solve with biomass gasification. The problems with biomass gasification aren’t technical, they are economic. According to the EIA’s Annual Energy Outlook 2006, capital costs are $15,000-20,000 per installed barrel for a conventional oil refinery, $20,000-$30,000 for an ethanol plant, around $40,000 for gas-to-liquids (GTL), around $60,000 for coal-to-liquids, and around $120,000-$140,000 for biomass-to-liquids.
Source: EIA Annual Energy Outlook 2006
The reasons for this should be obvious – it is much more difficult to handle biomass than to handle natural gas, for instance. Until we are willing (or forced) to pay a penalty for using fossil fuels, or are willing to pay a premium for renewable energy, biomass gasification is going to be passed over in favor of lower capital options. In the long-term, though, biomass gasification has staying power as an option for using biomass as a transportation fuel.
Vinod Khosla and Kergy
What actually prompted my interest in writing this essay were the media reports of Vinod Khosla’s latest alternative energy venture. This has been hailed as a breakthrough in cellulosic ethanol. While some may consider this a subtle distinction, I think it is very important that people understand the difference. It may make sense to preferentially fund gasification options over cellulosic ethanol options, but this will be more difficult if the public doesn’t understand the difference.
A recent entry in Venture Beat brought Mr. Khosla’s new venture to my attention. The company is called Kergy, and details were discussed in a recent story in Wired written by Vinod Khosla. Mr. Khosla explains:
In the corner of an unmarked warehouse tucked away in an industrial neighborhood north of Denver, a new company called Kergy has what is, to my knowledge, the first anaerobic thermal conversion machine (which explains why Khosla Ventures is a seed investor). It’s a 6- by 4-foot contraption that stands about 8 feet high. It looks vaguely like a souped-up potbellied stove. But it runs cleanly enough to operate indoors.
Kergy’s machine is special because it makes cellulosic ethanol through anaerobic thermal conversion rather than through fermentation or acid hydrolysis. It does not need organisms or enzymes to do its work. Biomass is heated in an oxygen-free environment to produce carbon monoxide and hydrogen. Once that happens, “the world is your oyster,” says Bud Klepper, the engineer who invented this device. The carbon monoxide and hydrogen are then reconstituted into various alcohols – like ethanol. Better still, fermentation and acid hydrol¬ysis can take days to occur, but thermal conversion breaks down organic matter and converts it to ethanol in minutes.
And here’s the really exciting part: Because all organic matter contains carbon, Klepper can make ethanol out of cellulose or any form of organic matter. This means the usual suspects such as corn, switchgrass, sugarcane, and miscanthus but also any waste product such as wood chips, paper pulp, cow manure, and even human waste. Municipal sewage has been tested already, as has hog manure. “We could double the ethanol output of the Mead facility,” Klepper says. It’s a big leap forward on the biohol trajectory, and it is right in front of us.
In back of Kergy’s warehouse, workers are busy putting the finishing touches on a beautified and expanded version of his original thermal convertor. The new one is made out of lustrous red I-beams, shiny metal tanks and coils, bright blue metallic joints, and a porous metal-grating floor. The whole thing is 14 feet high, 40 feet long, and 25 feet wide and is capable of producing 15,000 gallons of ethanol a day. And the machine can be scaled for far more capacity.
I knew this technology has been around for a while, so I looked up Klepper’s patents. After reading through the claims, it wasn’t at all clear to me what differentiated Klepper’s version from the patents that came before. Sometimes it boils down to very subtle differences in the claims, so I wrote to Mr. Khosla asking for some information:
Hi Vinod,
Just finished reading the Wired essay. Of course I disagree with several of the things you wrote, but that isn’t the purpose of this e-mail. What I am particularly interested in are the claims on “anaerobic thermal conversion.” Some people have been calling this cellulosic ethanol, but that’s really a misnomer because it is a completely different process. It is actually biomass gasification to produce syngas, a technology that has been around for at least 30 years. So it certainly isn’t “the first anaerobic thermal conversion machine.” Lots of people have done this, just not commercially. The technology for turning the resulting syngas into methanol, ethanol, or even diesel (via the Fischer-Tropsch reaction) has also been around for many years. As I am sure you know, the reason this hasn’t been done commercially before is the high capital costs per barrel of product. But I just did a patent search, and saw that Klepper has been issued a patent on the process. It just isn’t clear to me what distinguishes his patent from those that came before. Do you know? I am not trying to downplay the invention; differences in patents are often very subtle. But I am trying to determine how his patent differs from all of the other biomass gasification patents.
I will say that I believe you are on the right track with biomass gasification. I have never had any concerns about this technology, and I believe that this is clearly the future. I just don’t know if it will be commercially viable without subsidies or mandates, because it is much easier (and far less costly) to do the same process with natural gas (GTL). But it is certainly more efficient to gasify biomass than it is to ferment it. I think you will find that it would be far more efficient to turn the syngas into diesel, but you might lose out on the subsidies. I guess if the government accepts this process as cellulosic ethanol, then maybe they would accept that product as biodiesel (which would qualify for the subsidies).
Sincerely,
Robert Rapier
He responded, but on the topic of Kergy he wrote “I am not interested in public disclosure of what we are doing at Kergy at this stage. Hope you understand.” Of course I wasn’t asking for proprietary information; I just wanted to know what distinguished this patent from previous gasification patents.
Again, my purpose here is certainly not to denigrate those involved with Kergy. In fact, if an opportunity hadn’t come up recently at work (see the note at the end), I would seriously consider working for them. Mr. Khosla and I have discussed this, and I was contacted over the weekend by one of their Senior VPs. I think what they are doing is definitely a step in the right direction, and I think it would be fun to be a part of it.
I just want people to understand that this is not brand new technology, so they shouldn’t think that the cellulosic ethanol problem has suddenly been resolved with a breakthrough. Biomass gasification certainly works, but it worked 20 years ago. It is just a capital-intensive process that has the problem of competing against lower cost (but unsustainable) gasification options.
Personal Note
I recently accepted an offer to take up a management position within my company in Aberdeen, Scotland. I will be responsible for 10-15 engineers in our Europe and Africa business unit. Most of the work will involve exploration and production projects in the North Sea, but the best draw of all is that my family and I love Scotland.
My report date is February 1, 2007, and I will be pressed for time between now and then. Therefore, my posting will be sporadic over the next few months. Hopefully, after I get settled in over there, I can start contributing again on a regular basis. I have lived in Europe before, and I am slowly archiving the essays on our previous trips at Traveling in Europe. I plan to keep this updated as we travel around Europe. I will continue to maintain the same Gmail address, so feel free to contact me there with questions or comments.

Great article.
You know that I am a big believer in syngas fermentation (SF) as opposed to the other cellulosic ethanol technology – enzymatic hydrolysis (EH).
One of the benefits is that SF facilities can be located near the sorting centers for municipal solid wastes (MSW) so that the trucking costs and tipping fees are substantially reduced because very little residual ends up being transferred to landfills.
Another benefit is that SF does NOT require batching or sorting of feedstock. In EH feedstock sits in a vat during conversion to its sugar brew and then the sugar brew sits in a vat during fermentation into ethanol. Not only lots of water involved but also lots of time – 4-6 days/batch.
On the other hand, SF feedstocks can be blended for higher yields – MSW mixed with tires, for instance. From insertion into the gasifier to extraction as ethanol takes less than 10 minutes in a continuous feed.
The big question about Kergy is what fermentation process do they use after gasification? Fischer-Tropsch? As you write, that is hardly revolutionary (see Germany circa 1920’s). Other companies cite catalysts, while BRI Energy uses syngas-eating anerobic bacteria.
One thing you left out is the co-generation of electricity. Syngas can be combusted to produce steam (creating carbon emissions – not a great option) or the gasifier and syngas heat can be used to produce steam for a net electricity gain.
Since coal can be gasified and turned into ethanol using the same methods I am wondering if all those new coal burning electric generators should be equipped with gasifiers instead – producing electricity and ethanol with near-zero emissions.
BTW – I first saw your title at the new BlogNetBiz site.
“ethanol produced from fermenting grain typically makes up only 15-20% of the solution”
Not when you buy it in the store.
I don’t want to underplay the power of the great venture-driven hype machine, but consider the very real possibility that the novelty (and patent) is not for the syngas process itself but a clever way of building the reactor which cuts the capital expense.
If the operating costs of a syngas process are, indeed, substantially lower than a fermentation process, then figuring out how to cut the capex would be a Very Big Deal.
(And calling it “cellulostic ethanol” doesn’t bother me. It’s just marketing–at the end of the day the inputs and outputs are the same.)
Very well written, and congrats on your new assignment in Scotland.
Kergy may be protecting some unique implementation wrinkle with trade secrets instead of patents, but it seems equally likely that Khosla is simply outside his circle of competence and being fooled by enthusiastic inventors using big words. He’s following the classic high tech VC formula — identify a trend then spread your bets among early innovators who talk a good game. But energy markets don’t care much about time-to-market and buzz, it’s $/btu that matters.
I have two questions on biomass gasification. First, have you seen a good evaluation of a biomass to liquids path compared to a biomass to electricity to PHEV path? Seems like the latter would have lower capital expense and at least as good of a “miles per ton of biomass” net yield.
Second, have you ever looked at Eprida (www.eprida.com)? Not so much their complicated schemes involving coal plant exhaust, but the simple idea of gasifying biomass and burying the char to achieve long-term carbon sequestration and improved soil productivity. It’s the only practical-sounding approach I’ve heard toward reducing atomspheric CO2 levels.
The big question about Kergy is what fermentation process do they use after gasification? Fischer-Tropsch?
Scott,
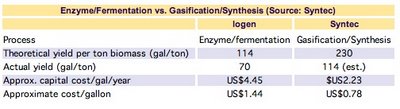
I believe it is going to be a chemical process following the gasification, sort of like what Syntec has been working on. I know that it is pretty easy to turn syngas into methanol or diesel, but the information on turning it into ethanol is pretty limited. Once you get to C3 and higher alcohols, the only way I know to turn syngas into alcohol is by adding an olefin (propylene, if you are trying to make butanol).
If they can do it chemically with decent yields, that is a better route than fermentation since you won’t have water to remove (and the reaction will be faster). The ideal alcohol to produce in this manner, though, would be methanol.
BTW – I first saw your title at the new BlogNetBiz site.
Yeah, I checked that out last night after you mentioned. That’s a good repository of information.
Cheers, Robert
First, have you seen a good evaluation of a biomass to liquids path compared to a biomass to electricity to PHEV path? Seems like the latter would have lower capital expense and at least as good of a “miles per ton of biomass” net yield.
I think you are absolutely correct, and I brought this up with Khosla. It would make the most sense, to me, to produce electricity from the biomass. Your overall biomass to transportation energy yield is going to be much higher. You could even start this process today by adding biomass to coal-based power plants.
Second, have you ever looked at Eprida (www.eprida.com)?
Had not heard of them, but sounds like the right approach. I will check them out. In the long-run, we are going to have to adopt an approach like this.
Cheers, Robert
First off, congrats on the new job offer, Robert. Well done.
This was a pretty informative post and I think should help many people get a sense for the difference between biomass-to-liquids gasification processes, and fermentation processes. Ultimately, I don’t have too much of a problem with the use of the term ‘cellulosic ethanol’ to describe any process that utilizes a cellulosic biomass feedstock and results in ethanol, but I agree that people need to have a clearer idea about the different kinds of technology that can produce ethanol from biomass.
I also agree that the biomass gasification approach is the better of the two, as it is significanty less ‘picky’ about feedstocks (fermentation processes usually require specialized pretreatment processes, enzymes and/or microorganisms for different feedstocks), can co-generate more useful steam and/or electricity, and utilize more developed technologies (technologies shared by the emerging gas and coal-to-liquids and IGCC technologies as well).
Scott Miller mentioned the BRI process, which I have researched a bit, which is an interesting hybrid of the two processes you describe. They gasify their feedstock but then feed the resulting syngas to a specially engineered bacteria that ‘eats’ the syngas and – to be both crude and simple – ‘craps out’ ethanol. They also co-generate quite a bit of electricity in the process (producing steam while cooling the syngas and running the steam through a steam turbine).
Ultimately, BTL or gasification-based cellulosic ethanol production has a lot of promise, but I think you hit the nail on the head that it will come down to costs. That is why, as the anonymous commenter pointed out, any incremental improvements in capital costs for BTL processes could very well be a big deal. Personally, I would argue for increased research spending to help make these processes cheaper and more efficient as well as subsidies to help BTL compete with fossil-fuel based GTL and CTL processes.
As for comparing a biomass-to-ethanol pathway to a biomass-to-electricty pathway (for EVs/PHEVs), I haven’t done that exact analysis, but I’m tempted to look into it. The electricity pathway could very likely be better, although clearly there are advantages to using liquid fuels as well – i.e., similar/same distribution infrastructure, vehicle engines, etc.
Robert said, “I have this neat new cellulose conversion process. I am looking for funding and working on a patent application.”
That’s a good’un Robert, but I think Ben Franklin beat you to it. However, I do believe if you get the right politician on your side, you can probably line up some tax credits or subsidies.
Seriously though, I’d like to see someone come up with a way to convert the billions of tons of leaves we are raking this autumn in the Midwest and Northeast into a biofuel. (I have so far raked about three tons of oak and maple leaves in my yard.)
The EROEI would be interesting though. It would take a lot of truck miles and diesel fuel to pick up all the downed leaves in the city I live in (Madison, WI) and transport them to a biofuel conversion plant.
Cheers,
Gary Dikkers
Robert, I was confused by this sentence:
“In fact, most syngas in the U.S. today is made from natural gas.”
Why would anyone make syngas from gas? Is the real objective to produce hydrogen?
Good luck in the new job!
Robert,
I reviewed the file history of Klepper’s patent to see how it was distinguished over the prior art.
Here is the first claim of Klepper’s patent (6,863,878):
1. A method of forming syn gas comprising devolatilizing a feed material in a controlled manner at a first temperature low enough to react oxygen in said feed material without causing pyrolysis thereby forming a char; subsequently combining said char with steam and passing said steam and char through a heated reaction vessel at a second higher temperature to form syn gas.
The key difference from the prior art (and what got the patent allowed) is the language “devolatilizing . . . at a first temperature low enough to react oxygen in said feed material without causing pyrolysis” followed by treating at a second higher temperature. The applicants argued during prosecution that
“Applicant’s invention is a very controlled system. The feed material is introduced into the devolatilization zones which are designed to cause available oxygen in the feed material to react with the feed material at a temperature less than 450° F which prevents pyrolysis. If one continues to add additional oxygen-containing gases into the feed mixture, it is not devolatilized. Pyrolysis is an exothermic reaction and most prior art syn gas production methods’have utilized the heat generated in this portion of the reaction to fuel subsequent gasification. Pyrolysis in the presence of oxygen causes the formation of slag. This is exactly what Applicant is trying to avoid.”
Use that for what it’s worth.
Why would anyone make syngas from gas? Is the real objective to produce hydrogen?
Most syngas is made via partial oxidation (POX) of natural gas. The syngas can then be used to make a wide variety of chemicals. With my former company, we used syngas to react with ethylene or propylene in order to produce propanol and butanol.
Most hydrogen is also produced from natural gas, either as excess hydrogen from a POX reaction or in a hydrogen reformer.
Cheers, Robert
The key difference from the prior art (and what got the patent allowed) is the language “devolatilizing . . . at a first temperature low enough to react oxygen in said feed material without causing pyrolysis” followed by treating at a second higher temperature.
Thanks for looking into that. One thing I noticed was that the reaction temperature was quite low relative to most gasification reactions. But I wonder if that means the reaction is not self-sustaining, and requires continuous inputs of energy.
Cheers, Robert
Here’s a GREET study of various switchgrass processing approaches by our friend Mr. Wang:
http://www.transportation.anl.gov/pdfs/TA/344.pdf
Syngas fermentation is not included. Cellulosic ethanol is teamed up with a couple different co-gen/co-product approaches and compared to a gasification/Fischer-Tropsch approach and a gasification/dimethyl ether approach. All are “advanced” processes, supplying all process heat/power from the feedstock and exporting some electricity.
The Fischer-Tropsch and DME approaches yield less liquid fuel but more electricity. They get penalized a little for this, but if the electricity fed PHEVs I think they’d both come out ahead. There’s probably enough raw data in this paper and the referenced literature to figure out how BRI’s process would compare.
Your link got chopped off. Here it is:
GREET Biomass Analysis
Cheers, RR
Robert,
Ulf Bossel from the EFCF slammed hydrogen and said it will never be the dominant carrier.
http://www.efcf.com/reports/E15.pdf
You should do a review of hydrogen even if it’s only to concur with Ulf Bossel.
This is a pretty good convo at The Watt as well.
Ulf Bossel from the EFCF slammed hydrogen and said it will never be the dominant carrier.
I certainly agree with that. I looked at hydrogen pretty closely about 4 years ago. There are so many enormous technical challenges to overcome that I don’t see the hydrogen economy as anything but a pipe dream. It’s one thing to solve a technical challenge, but we are talking about 4 or 5 quantum leaps with no clear way to get there. It looks about as viable as human colonies on Venus.
Cheers, RR
Heh, it looks like you guys have very different opinions.
he says:
Without the slightest doubt, the technology for a hydrogen economy exists or can be developed in reasonable time. Also, hydrogen is an appropriate energy carrier for particular niche applications, or it may become an important medium for electricity storage with reversible fuel cells. But hydrogen can never establish itself as a dominant energy carrier. It has to be fabricated from high grade energy and it has to compete with high grade energy in the marketplace. Hydrogen cannot win this fight against its own energy source.
http://www.efcf.com/reports/E15.pdf
You claim technical challenges but he claims lack of cheap hydrogen.
Heh, it looks like you guys have very different opinions.
A few years ago, I read a DOE report on the hydrogen economy. I still have it around here somewhere. I think the title was “Road to the H2 Economy.” In describing the challenges, they identified a number of areas, and indicated where we are now, and where we needed to be before this could become reality. What struck me is that they showed a number of graphs, in which the status quo had a number of data points on a graph centered around low performance, high cost. Where they needed to get to was high performance, low cost. But there weren’t even any intermediate points; say high performance, high cost. Because this was the case with 4 or 5 critical areas, I decided at that point that the H2 economy is a remote dream at the present.
The biggest problem is one of chemistry and physics. The energy density is simply too low. This affects storage, both in a vehicle and at a station, and transportation of the hydrogen. Furthermore, fuel cell vehicles are incredibly expensive, and dependent on rare metals. Finally, hydrogen itself is presently produced mostly from natural gas. You could produce small amounts from excess electricity, but rolling out a mass solution is going to require incredible amounts of electricity (or natural gas) and it will put pressure on those rare metals used in the fuel cells.
Cheers, Robert
Robert,
I am a bit confused why ethanol has become such a buzz word. Suddenly it seems like “ethanol” is a synonym for “renewable energy”. One only has to read up on the practical challenges faced by E85 to understand that ethanol is no silver bullet. In a gasification system, I agree, it would make more sense to produce F-T diesel. You can call it renewable diesel and get a $1/gal subsidy (as CWT does with TDP), or about double the ethanol subsidy on a volume basis.
The BRI process, while interesting, in my mind, does not make a lot of sense. Why ferment syngas, and get a broth that requires energy-intensive distillation, when you can go straight to F-T diesel (and from there to other common hydrocarbon fuels)?
On the scalability issue: have you seen the Velocys System. Of course, they had to call it nanotechnology. As I see it, the Velocys System can be used for gasification of gas and F-T. That leaves gasification of biomass as the remaining challenge.
Well, in some applications, I guess one could use anaerobic digestion (as in municipal wastewater) to convert the biomass into biogas, which can then be fed to a Velocys gasification/F-T system.
Look forward to your thoughts.
The BRI process, while interesting, in my mind, does not make a lot of sense. Why ferment syngas, and get a broth that requires energy-intensive distillation, when you can go straight to F-T diesel (and from there to other common hydrocarbon fuels)?
That is my position as well. If you have syngas, you really want to avoid making a product that you have to take water out of. Either turn it directly into methanol or FT diesel. If there were no ethanol subsidies involved, this would be a no-brainer.
On the scalability issue: have you seen the Velocys System. Of course, they had to call it nanotechnology.
Yeah, the nanotechnology reference was priceless. That word has become so overused it is meaningless. I could call a tube of toothpaste nanotechnology with just as much justification.
What they describe is an XTL process. My guess is that they will start with GTL, because it is the cheapest system to operate. But it isn’t cheap. I spent a couple of years running a GTL lab, and I am pretty familiar with the economics and the process. What they are getting funded for has been done, by lots of different companies.
Cheers, RR
Interesting post.
Let me add a few thoughts by answering a question,
why do we want to convert biomass into liquid fuels, and what options have we got?
Biomass can be converted to electricity and, at least in principle, be used to power electric cars. There are issues with batteries, but leaving that aside, we might want liquid fuels anyway, say to power planes or plug-in hybrids,
and we are still left with the question of how best to obtain those liquid fuels.
There are two main approaches:
1. Get algae or trees to produce hydrocarbons directly => Main drawback being low yield at present, or very high capital cost and/or inputs into growing
2. Make do with what we do get the best yields for at present: lignocellulosic biomass.
We can then either go for thermochemical or biological conversion, or a combination of the two, say a biorefinery where lignin is turned into petrol through Fischer-Tropsch and cellulose is turned into ethanol via enzymatic hydrolysis.
Ideally of course we’d like to find a catalyst/bacteria that’ll turn near 100% of the biomass into a hydrocarbon at room temperature in low capital cost equipment.
Sugar does get turned into ethanol at greater than 97% efficiency by bacteria, at room temperature and in relatively low capital cost equipment.
But as you say, it’s then in solution, and because of all the water around it, we’d want to separate out the water. That is currently quite energy intensive.
If that can be resolved at acceptable cost, say using molar sieves, converting sugar to ethanol would indeed be hugely efficient (97% is so close to a 100, there wouldn’t be much point trying to get even better).
And if cellulose could be converted at low capital and enzyme cost into sugar, this would appear to be a very attractive proposition (Cellulose to sugar to ethanol at greater 97% efficiency).
The energy cost of separating out the water is the same for corn ethanol, cellolosic ethanol and sugar cane, more or less anyway.
In net energy analysis this is hidden away somewhat, because we don’t look at total energy in (corn +natural gas say) over total energy out (ethanol say), but rather at fossil fuel inputs compared to ethanol output. For sugar cane and corn ethanol, conversion efficiency on a total inputs basis is quite comparable, but the energy for distillation is supplied by burning bagasse rather than natural gas.
But that doesn’t mean that the distillation energy use wouldn’t be there. It still needs to be addressed.
If we don’t do it via zeolite membranes (or similar ways of enhancing ethanol/water separation), and we can’t do it via fermenting to a higher alcohol or directly to a hydrocarbon (the less oxygen there’s left in the liquid fuel, the more easily it’ll separate from water),
we can instead go the thermochemical route. Even if we produce an alcohol that way, at least it’s not contaminated with lots of water.
Here the main possibilities are syngas production followed by Fischer-Tropsch, and direct hydrogenation with hydrogen produced from part of the biomass:
CH2O goes to CO2 + H2
and then
CH2O + H2 goes to CH2 + H2O
The efficiency of either appears to be around 40%.
What I do not quite understand is what the limitations there are, ie why the energy is lost, and how fundamental the problems are.
Obviously, as pointed out above, in theory, we could find catalysts over which biomass will turn into hydrocarbon at room temperature and near 100% energy yield. After all sugar to ethanol is pretty close, with the catalyst being the enzymes within the fermenting microorganisms (if only they could do the trick without needing all that water, and even better taking the sugar contained in cellulose).
Robert,
Would you care to take a shot at the EROEI of a BTL process? If the gasification step is self-sustaining, and the dehydration of the ethanol is elminated, then that suggests that the EROEI could potentially be huge. The major energy inputs would be the production and transportation of the biomass, and if you’re farming a fast-growing shrub or tree and feeding woodchips to an on-site production system, you could eliminate most of those.
I well understand that there are important metrics besides EROEI. But it would be interesting to know that there is a process for producing liquid fuels with currently available technology that has an EROEI on par with petroleum production, if that is indeed the case.
Thanks for looking into that. One thing I noticed was that the reaction temperature was quite low relative to most gasification reactions. But I wonder if that means the reaction is not self-sustaining, and requires continuous inputs of energy.
Perhaps it is easier to divert some of the product and burn it outside the reaction chamber to provide the required energy, than to deal with the slag that would result from running the main reaction at a higher temperature.
New Georgia Ethanol Plant Turns “Waste to Value”
Did you have a chance to look at this claim Robert?? Perhaps they are doing the same thing as Kergy??
Did you have a chance to look at this claim Robert?? Perhaps they are doing the same thing as Kergy??
From reading about the process, it sounds very similar to a Kergy process. One of the issues that will be of interest to watch is that I know someone who says he holds the patents for mixed alcohols, and that is exactly what they are going to get. So there may be some litigation before it’s all over with, which I had not anticipated.
Cheers, RR
Hi everyone,
I am doing a research project on biomass gasification and I agree that it is a very promising technology. However, there seems to be a lot of different technologies even within the gasification process itself, and I am just wondering what everyone’s opinions are on which is the most promising. For example, these are just a few of the technologies that I’ve come across:
-Alchemix/Diversified Energy has their own process called Hydromax that can take any carbonaceous feedstock and react it in a molten iron/tin bed to make hydrogen and syngas in separate streams (search for Alchemix in US Patent office website under assignee name to see their 4 patents)
-Various companies use plasma arcs that turn any carbonaceous material into syngas
(eg. Startech, MPM Skygas, etc.)
-Choren’s Carbo-V process that makes F-T Diesel
-Range Fuels uses the Klepper Pyrolytic Steam Reformation which they claim to be the most efficient system for gasifying biomass
Your Opinions would be much appreciated!
Slag formation is certainly an issue but not on par with the problem of tar formation from what I understand. A major issue with biological fermentation to ethanol is the poor conversion of biomass. Sure sugars can be fermented at near 100% efficiency, but a low cost biofeed stock is going to convert at between 40 and 60%. A huge waste of material. A combined bio and thermochem approach such as that of Zeachem may work well although I am not sure they can produce a high enough concentration of acetic acid per batch to avoid high capital expenses.
Hi Everyone,
I am not an energy nerd, but I am a little surprised when syngas per se can be used in ICE as well as gas turbines, Why should anyone think of ethanol at all?
I am trying to convert a diesel into SI for syngas application, any help is welcome.
Robert,
There is nothing new under the sun – regardless of what clever terminology might be tagged to it.
Here is biomass gasification circa 1913, using processes that were well understood by 1850
http://www.nelmes.fsnet.co.uk/paxman/suctngas.htm
Scroll down for the section on “Special Fuels”
The current obsession for ethanol for gasoline substitute has reached fanatical heights.
Anyone who realises the energy input requred to ferment and then distil alcohol into fuel grade ethanol, must surely realise when it’s time to quit and use the hydrocarbons in a more direct manner.
Our grandfathers knew when to cash in their chips – as per this excellent 1903 publication on Mond gas.
http://www.archive.org/details/mondgas00woodrich
The Mond process could equally be applied to biomass as it can low grade coal.
http://www.archive.org/details/mondgas00woodrich
An excellent read – even our great-grandfathers had a very strong handle on EROEI and valuable coproducts.
As an aside, to harvest sufficient biomass and transport it economically to gasification plants we are going to have to forget trucks and use railways.
If we have to cover the corn belt from Texas to Canada with a network of efficient electric railways, run from biomass fuelled generation stations – so be it.
This would be no different to the use of railways for transport logistics in the American Civil War and WW1 and WW2.
However, the above mentioned conflicts resulted in the mechanised anihilation of humans, and further exploitation of the corn belt will result in the systematic destruction of the remaining topsoil.
Biomass, w/o Cellulosic conversion, if done right, and there are lots of variables, crop, fertilizer, etc., can produce almost 4,000 gallons of diesel/ acre equivalent. Unfortunately folks have invested heavily in Jatropha (which is poisonous if you eat the seeds). Associates has plantations approaching this level of production. JRIAM1945@AOL.COM
Great Article..
Although i am not a science student but these recent development in BioFuels has developed my interest in this field. Our company plans to diversify into Biofuels with the help biomass gasification, as it appears to be more cost-effective than cellulosic process (correct me if i am wrong)
1.) Does anyone have a vague idea about the conversion from biomass to ethanol through gasification? The cost of production in India will vary a lot then what i find in many articles.
2.) Any suggestions on good contract-researchers or where we can look up for technology transfer ? as india lacks a good resource infrastructure.