Update: E3 Biofuels, with their promising ethanol technology, has declared bankruptcy.
I hope it is clear that my opposition to ethanol has nothing to do with the fuel itself. If we could make sufficient ethanol with little or no fossil fuel inputs, ethanol could be a very important piece of a post-petroleum future. If ethanol could be produced with an EROEI of 3 or 4, as opposed to the current 1.0-1.3 or so, then ethanol begins to look attractive from a sustainability standpoint. My opposition to ethanol is due to the way we typically make it in the U.S., and is specifically focused on grain ethanol. We take fossil fuels and basically recycle them into ethanol in a very inefficient manner. Ethanol production may be a good solution for countries like Brazil, that don’t rely on large fossil fuel inputs into the process (as long as they aren’t depleting their topsoil and cutting down their rainforests). Cellulosic ethanol may ultimately provide ethanol at a substantially better energy return than grain ethanol, but as a recent Car & Driver article put it: “If cellulosic ethanol were easy, it would already be on the road, because the government has been seriously funding research for about 30 years.”
There are even some places in the U.S. where ethanol could provide a (mildly) sustainable solution even as it is produced today. Take Iowa, for instance. Iowa has good corn yields and doesn’t require irrigation. If the ethanol is produced from local corn, and is used locally (not shipped halfway across the country), the renewable portion of ethanol is increased. This may provide marginal mitigation for peak oil in certain local areas (though it is still not a highly efficient way to produce fuel). But get into areas outside the Midwest, where you have to ship corn a long way, ship ethanol a long way, and/or irrigate the corn, and ethanol rapidly becomes just a recycled fossil fuel. However, a couple of months ago a poster referred me to a company that is attempting to produce ethanol in a more sustainable manner. The company is E3 Biofuels. Their concept is this: Grow corn, produce ethanol, feed the byproducts to cattle, harvest the manure, produce methane from the manure in a biodigester, use the methane to fuel the boilers, and use the remaining solids to fertilize the soil. This is ethanol production in more of a Brazilian mold (i.e., byproducts are used to fuel the process).
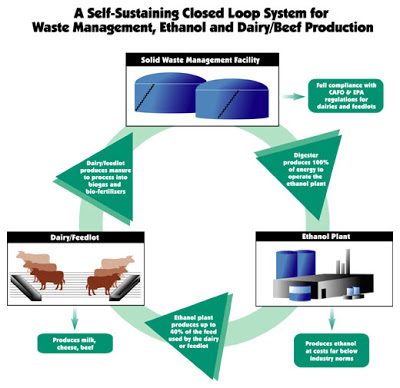
E3 Biofuels’ Closed-Loop Ethanol Process
I had some skepticism about whether they could produce enough methane to completely provide the energy needs of the plant, so I contacted the company. I was sent a spreadsheet from one of the project managers that estimates the energy inputs and outputs of the plant. I was correct that supplemental natural gas will be needed, but due to the manure-produced biogas, the amount is estimated to be substantially less than for a typical grain ethanol plant. Based on the spreadsheet I was sent, as well as correspondence I had with the project manager, the fossil fuel usage is estimated to be 75% less than that of a standard grain ethanol plant. If these estimates turn out to be accurate, that would quadruple the EROEI of the process. The project manager with whom I corresponded indicated that while there are a number of animal waste digesters, their facility will be the first to use the biogas in a closed-loop process. It is important to note that the process has not yet been proven. They are in the final stages of constructing their facility near Mead, Nebraska. They expect to finish the facility soon, and should be producing ethanol by September 1st. Their location has one disadvantage, however, and that is the need to irrigate corn in Nebraska. That means that the overall EROEI would not be as high as for a facility built in Iowa or Minnesota, for instance. If the process works as advertised, the EROEI could reach 4 or 5 to 1, or even higher for the same process in Iowa where corn irrigation is not required. Clearly, as I have argued in the past, ethanol is not going to lead us to energy independence. We simply don’t produce enough corn for grain ethanol to be a large part of the solution (it will certainly be no more than 20% by volume of our current fuel usage), and therefore conservation is going to have to make the biggest contribution toward sustainability. But if ethanol is going to be part of the solution to diminishing oil supplies, E3 Biofuels is the first in the U.S. to show the way toward making ethanol in a more sustainable manner. As natural gas supplies diminish, many ethanol producers are turning to coal as a fuel source. E3 Biofuels, on the other hand, may become the poster child for clean, “green” ethanol. As a long-time ethanol skeptic, the approach by E3 Biofuels is the first U.S. grain-ethanol process that I endorse.
RR, as a chemical engineer, perhaps you can comment on some of the issues relevant to ethanol, regardless of the source, i.e. octane number, hygroscopic nature, corrosion, vapor pressure (neat and in gasoline mixtures).
In other words, based purely on the properties of ethanol, is it an option worth pursuing, or are we better of looking for a better fuel?
From a scientific viewpoint, I think butanol would be a better option. According to the guys at http://www.butanol.com , butanol can be made from corn with the same volume yield and at similar cost. Yet it has similar octane, 30% higher BTUs, is not as hygroscopic, has a lower vapor pressure (which is a problem with ethanol in warm climates), reportedly can be blended in diesel or gasoline, and can be sent through pipelines.
However, this is not an entirely scientific issue. Based on the politics, grain ethanol is not going away. There is too much invested, and I think grain ethanol producers will lobby to prevent butanol from taking away market share. So, facing this reality, I think it is important for companies like E3 Biofuels to be rewarded for trying to do it right.
RR
Fair enough. I find it hard to believe that butanol can be as good as is claimed. As you allude to, the same volume yield would mean than butanol fermentation is 30% more energy efficient.
We have been doing ethanol fermentation since the dawn of civilization. Over thousands of years the fermentation process has been continuously improved. You want to tell me two guys in a garage came up with something that is 30% better, pretty much overnight?
To put this in context, E3’s process would give them an EROEI roughly on par with the low end of modern oil production from e.g. a stripper well or a well running heavy water injection. Does that sound about right?
That’s not actually a criticism: I’m thrilled at the idea of a biofuel with an EROEI remotely on par with petroleum.
On the other hand, I’m concerned about the natural capital investments that aren’t part of the EROEI equation, specifically water and soil. Conventional corn production is really hard on the soil. And organic corn is probably too expensive to use for fuel. On the other hand, they are putting manure back on the fields, so that helps.
R^2: does the spreadsheet they sent you extend to dicussing the nutrient balance in the corn cultivation process? I’d be curious to know how much of the needed nitrogen is coming from the manure vs. artificial sources. This obviously has an impact on the energy balance, but I’m actually more interested in the implications for soil health: if they get a large fraction of their nitrogen from manure, that implies that they are adding back alot of organic matter, which is critical to soil health and erosion control and thus to long-term productivity.
Re Butannol vs Ethanol:
Ethanol is C2H5OH. Butanol is C4H9OH.
C2H5OH + C2H5OH = C4H9OH + H2O.
How much energy would that reaction cost? and does the result need to be distilled to remove water?
We have been doing ethanol fermentation since the dawn of civilization. Over thousands of years the fermentation process has been continuously improved. You want to tell me two guys in a garage came up with something that is 30% better, pretty much overnight?
But we haven’t been doing this for fuel for all that long. Butanol is not drinkable, so it is not surprising that ethanol fermentation goes back much farther. The physical properties of butanol certainly make it a more attractive fuel. I think the question is whether it can be made as cheaply.
RR
does the spreadsheet they sent you extend to dicussing the nutrient balance in the corn cultivation process?
Yes. There is a co-product section that defines nitrogen, P2O5, and K2O outputs. If I am reading it correctly, the only shortfall will be on nitrogen, but the manure will provide around 25% of the nitrogen fertilizer needs of the corn. P2O5 and K2O are produced in excess of what is needed.
RR
Ethanol is C2H5OH. Butanol is C4H9OH.
C2H5OH + C2H5OH = C4H9OH + H2O.
How much energy would that reaction cost? and does the result need to be distilled to remove water?
You definitely wouldn’t want to make butanol like that (from ethanol). The reaction pathway is direct when it is made from microorganisms. Yes, you have to distill to remove water, but butanol is only partially soluble in water. It should be much less energy intensive to separate butanol from water.
In fact, I used to be the process engineer for a butanol distillation unit, and I need to figure up what the distillation energy amounted to compared to ethanol. My bet is that it is signficantly lower per BTU of butanol produced.
RR
“You definitely wouldn’t want to make butanol like that (from ethanol).”
OK. I’ll bite. Why not?
OK. I’ll bite. Why not?
It would be incredibly inefficient. You would have energy losses in each production step. Burning the butanol you produced would provide far less energy than burning the 2 ethanol molecules you used to produce it.
In the process described at http://www.butanol.com, which was invented by an Ohio State professor, microorganisms make butanol directly (just like some microorganisms make ethanol). It is just that butanol is more suitable as a fuel than is ethanol.
RR
C2H5OH + C2H5OH = C4H9OH + H2O
I am no organic chemist, but I don’t think you can do that. Just because you can write it on paper does not mean you can actually do it. I believe under suitable conditions you would get the following:
C2H5OH + C2H5OH = C2H5OC2H5 + H2O,
in other words you’d end up producing diethyl ether, not butanol.
Hmmm. Maybe I’m misunderstanding here, but couldn’t you achieve a better EROI by simply burning the waste to generate electricity, rather than go through the effort of putting it through a cow, collecting the cow poo, and running that through a biogas digester?
Maybe I’m misunderstanding here, but couldn’t you achieve a better EROI by simply burning the waste to generate electricity, rather than go through the effort of putting it through a cow, collecting the cow poo, and running that through a biogas digester?
The waste has a very high water content, so probably not. But it wouldn’t help them much in the process, because process steam is their biggest need. The most efficient way of producing process steam is from burning fuel – in this case the fuel they made from digesting the wastes.
I would also add some comments that I received in an e-mail from the Project Development Manager. He wrote:
Couple of points I would make. First, not all of our biogas comes from the animal manure. In fact, about 65% of our biogas is produced from the thin stillage (which is a byproduct of the ethanol process). This stillage produces more biogas per lb/VS than does manure and we have more thin stillage going into the digesters than we do manure.
Second, we do not use biogas to heat our digesters. They are mesophilic and run at 96 Degrees Fahrenheit. The hot thin stillage coming out of the ethanol plant is what keeps the digesters at this temp.
Also, though at this point we only can safely say that we use 75% less Fossil Energy than a typical ethanol plant, I guarantee you that we will get to 95% less before it is all said and done. We will build new facilities in the future and we will have monumental efficiency gains. My 75% number is my safe conservative figure.
Finally, we separate the digestate (after the solids have been completely volitalized) into N,P,and K. These are then returned to the soil.
RR
I’d ask how they propose to separate the N, P and K without expenditure of energy.
It occurs to me that a solar assist to the stills could eliminate the remaining gas consumption and even allow cogeneration with the bio-gas.
Hi Guys,
Re. Oil Peak, I have a question, that seems to fit in this forum…
I recently heard abt this Fischer-Tropsch technology where you can produce diesel or gasoline from coal, and was surprised I never even heard of this before, even though it’s been in industrial use since WW2.
The info I got was that you can produce a barrel of “ultra-clean” diesel/gasoline for ca US$35, and South-Africa already used it for decades during the Apartheid oil blockade, to produce the bulk of their tranportation fuels. Also, using this method, US has enough coal-reserves to meet current demands of transportation fuels for more than 2 centuries.
From the above data, this seems like the obvious Oil Peak solution for the to me, but are they correct?
What is your input on this?
What are the drawbacks?
The energy balance I have no info about — do you know how good/bad it is?
Taylor,
The energy return on a coal to liquids process is pretty good, but the capital costs per barrel of liquid produced are very high. That’s why CTL is not making a big splash.
Re: Coal to Liquids (CTL):
At $6.5B and 5-7 years construction time for 80k/day, you get a capital expenditure premium of $15-20/barrel, which is not bad given that you have diesel that sells for more than $80/barrel (i.e., no refining step like for oil). The problem is the enormous risk of such a large project with such a long lead time – the lead time is longer than the longest oil futures contract, making hedging difficult.
Here’s an article that promises lower expense and lead time:
http://www.morningjournalnews.com/include/articles.asp?articleID=3536
They say $4B and 4 years construction time for 96k/day. That’s a big improvement, and gives a capital expenditure premium of $9.20 at 7% interest, or less than the cost of refining oil into fuel.
CTL is terrible for global warming, but I suspect it’s coming faster than we think. It’s time for our society to make a commitment to efficiency improvement & electrification of transportation and renewables for electrical generation. Sadly, given how cheap coal is, to do it we’ll have to make a commitment to paying a premium to prevent Global Warming, which we’re not ready to do.
BGT Biogasoline might have a better option than ethanol. Ferment sugar to butyric acid, convert butyric acid to hexane using Kolbe electrolysis, and hexane to hexane isomers (such as dimethylbutane, which has a 93 octane). Thoughts?
bnThoughts?
At first glance, and without seeing an energy balance, the electrolysis sounds pretty energy intensive. Has someone actually developed a process along these lines?
Thanks,
RR
Hmmm. Maybe I’m misunderstanding here, but couldn’t you achieve a better EROI by simply burning the waste to generate electricity, rather than go through the effort of putting it through a cow, collecting the cow poo, and running that through a biogas digester?
Interestingly enough PANDA, a company in the panhandle of Texas is doing just that. Instead of putting manure through digesters, they have a biomass boiler, where they burn the manure to produce steam to provide energy for their ethanol plant.
The waste has a very high water content, so probably not.
Manure can be easily and efficiently dewatered with screw presses. They do it all the time.
But it wouldn’t help them much in the process, because process steam is their biggest need. The most efficient way of producing process steam is from burning fuel – in this case the fuel they made from digesting the wastes.
They can also burn the manure directly to make steam as manure a is FUEL. Again, PANDA is doing just that.
SO E3 and PANDA are the two companies that are making conventional corn ethanol a little more energy efficient, improving their EROEI. The former makes methane from manure and the other burns the manure directly.
Many regards,
CG
good site… how do you manage so many visitors on your site
good site… how do you manage so many visitors on your site
It was easy once I decided to give up sleep. 😉
Cheers, RR
Looks like E3 is in chapter 11 now see http://www.chapter11library.com